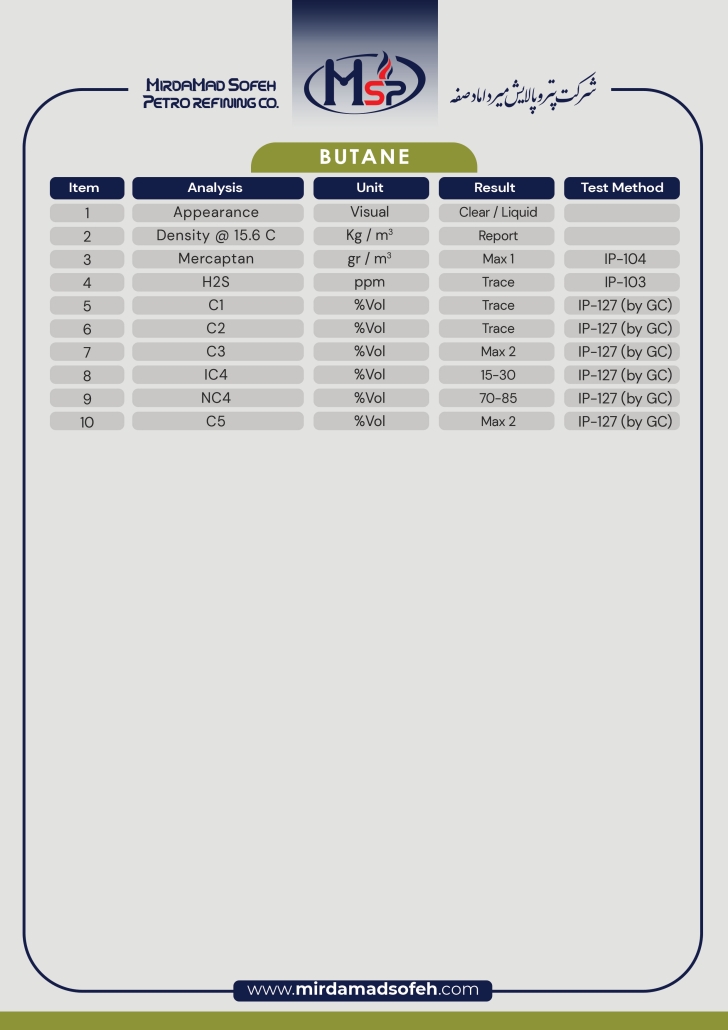
Butane
Butane (C4H10) is a flammable, colorless, gaseous hydrocarbon with a mild odor similar to natural gas or gasoline. It exists in two forms, isobutane and normal butane, and is usually stored and transported as a pressurized liquid (LPG). Butane has a wide range of applications in various industries due to its specific chemical and physical properties, including as a fuel, a raw material in the chemical industry, and a solvent in some processes.
Physical and chemical properties of butane
Flash Point: The flash point of butane is about -60 °C. This temperature indicates the high flammability of butane in the presence of a spark or flame source, which is important for safety in use and storage.
Boiling Point: The boiling point of butane at standard pressure is about -0.5 °C (for normal butane) and -11.7 °C (for isobutane). This property makes butane readily gassed at room temperature unless it is kept under liquid pressure.
Solubility: Butane has very low solubility in water (about 61 mg/L at 20°C), but it dissolves well in organic solvents such as alcohol, ether, and other hydrocarbons. This property makes it a suitable solvent in some chemical processes.
Oxidation resistance: Butane is stable under normal conditions and has good resistance to oxidation. However, it can react rapidly and burn in the presence of oxygen and a spark, which requires safety precautions.
To order this product or other products and receive a sample, you can contact Mirdamad Sofeh Petrorefining Company.